Electronic Batch Records (EBR) Software
Digitalise your reporting with paperless records and logbooks. Produce safely and in accordance with ALCOA+ and GMP frameworks.
What is Electronic Batch Records (EBR) Software?
EBR system or Electronic Batch Record system is a software solution for manufacturing facilities that are required to maintain safety standards and monitor the production process with precision. It allows to digitalise of the entire paper-based batch records, enables automatic data acquisition from machines, and creates gives a possibility to establish automated workflows with specific conditions tailored forms for each product. EBR system is commonly used in food, cosmetic, pharmaceutical other life sciences industries to track and trace goods manufacturing.
The common benefits of EBR software.
- Execute paperless goods production
- Validate production and eliminate errors by automatic data acquisition
- Incorporate complete manufacturing traceability
- Generate detailed report on the production of a selected batch
- Analyse production performance monitoring
- Satisfy GMP, GAMP and other manufacturing norms
What are Electronic Batch Records?
Electronic Batch Records (EBRs) are digital documents for tracking batch production data. The records contain information on dates, type and quantity of ingredients, equipment used and personnel present during the production of the selected batch. This information is essential to demonstrate regularity in the event of an inspection. Get more information about electronic batch record, or master batch record.
How EBR system can work for you in your plant?
1.
Use workflow planning tool to model MBR documentation flow
- Build a dedicated workflow for a product or product group
- Assign a list of tasks to be performed at any stage of the process
2.
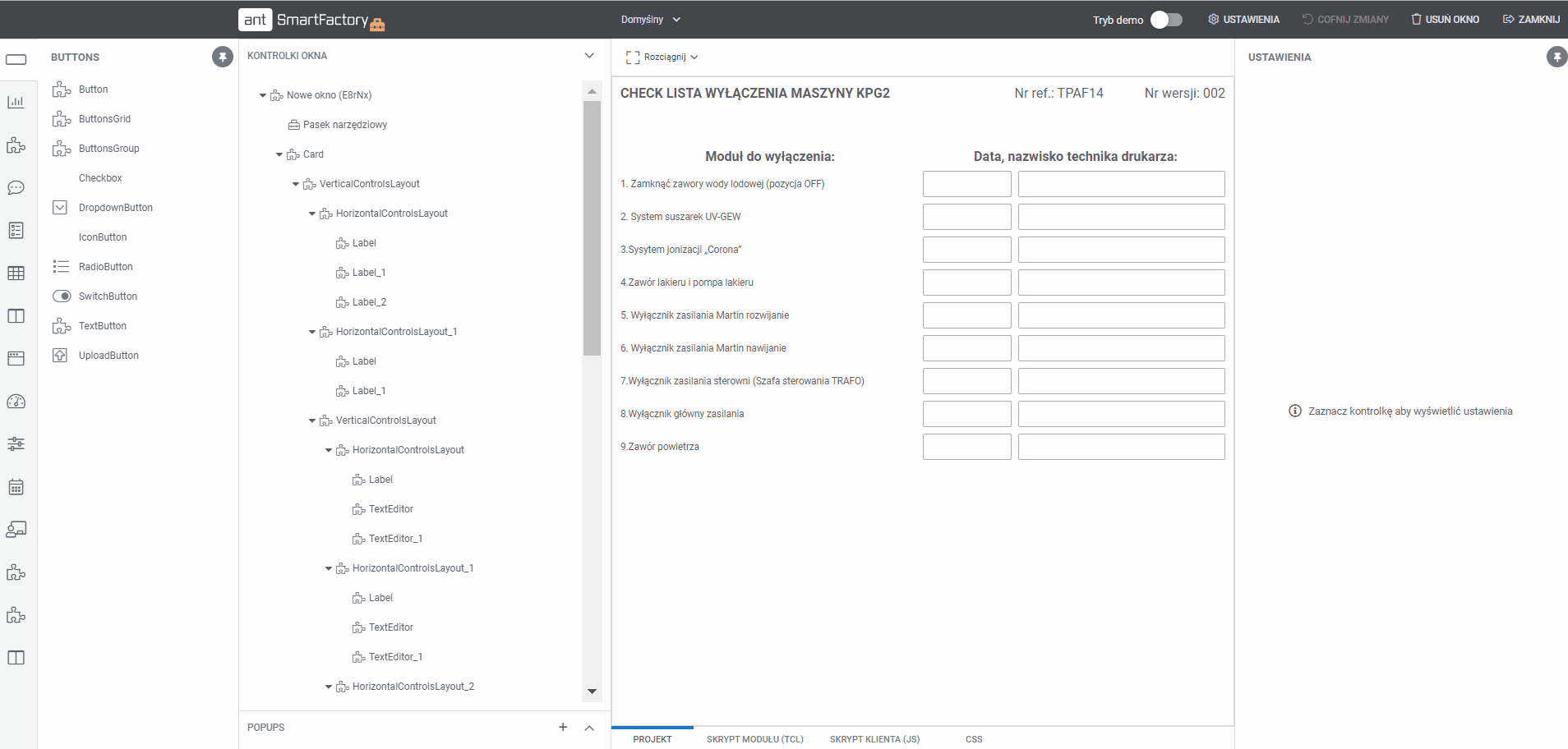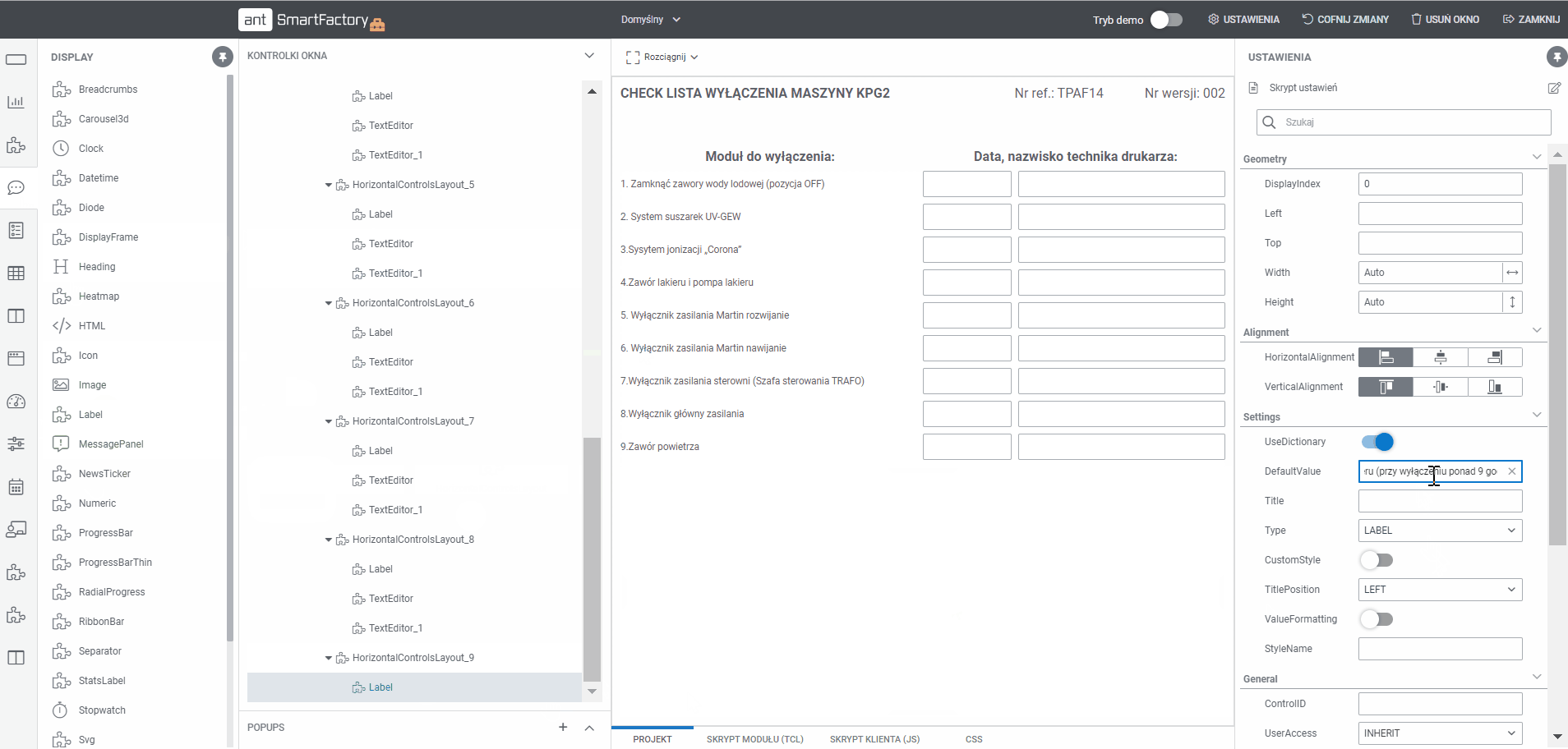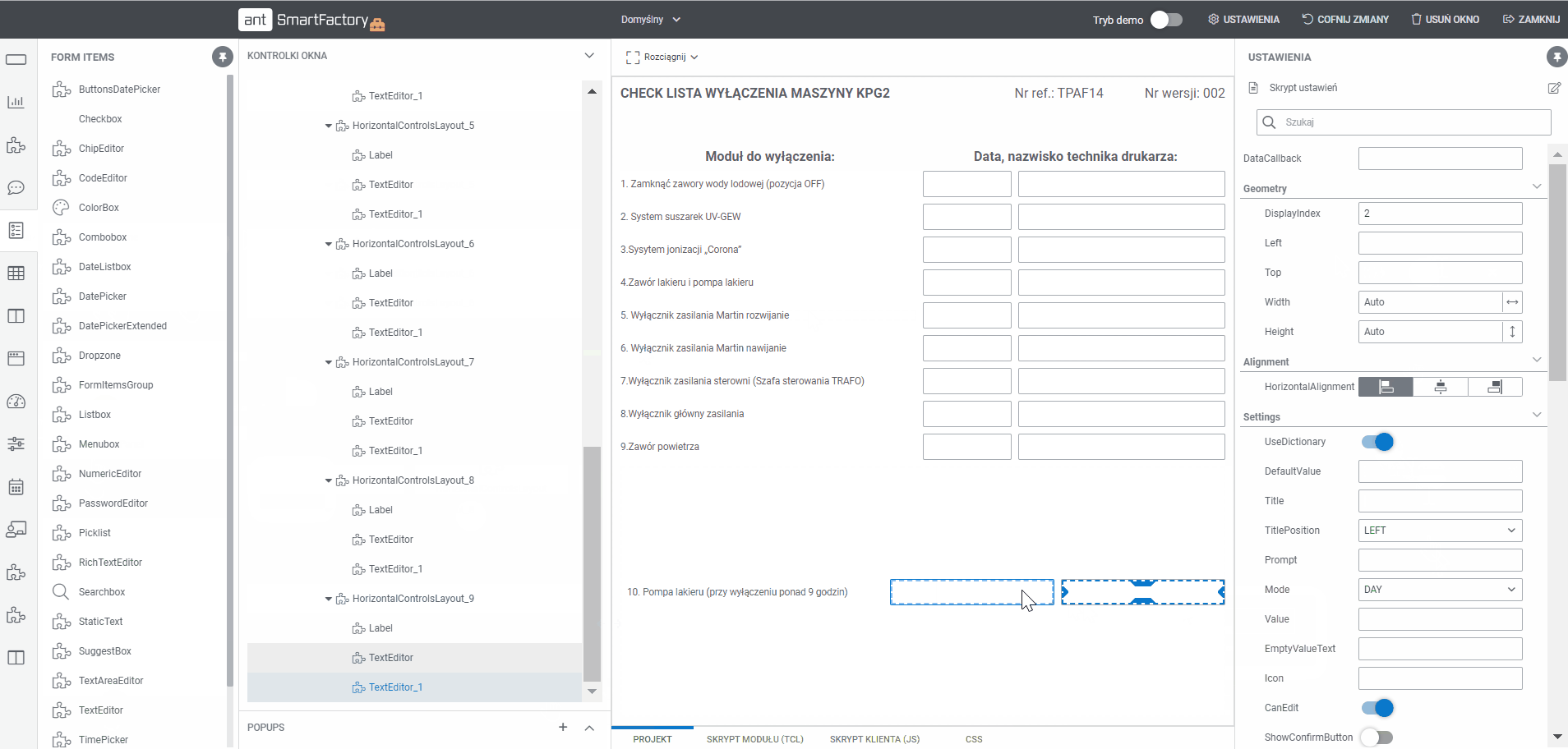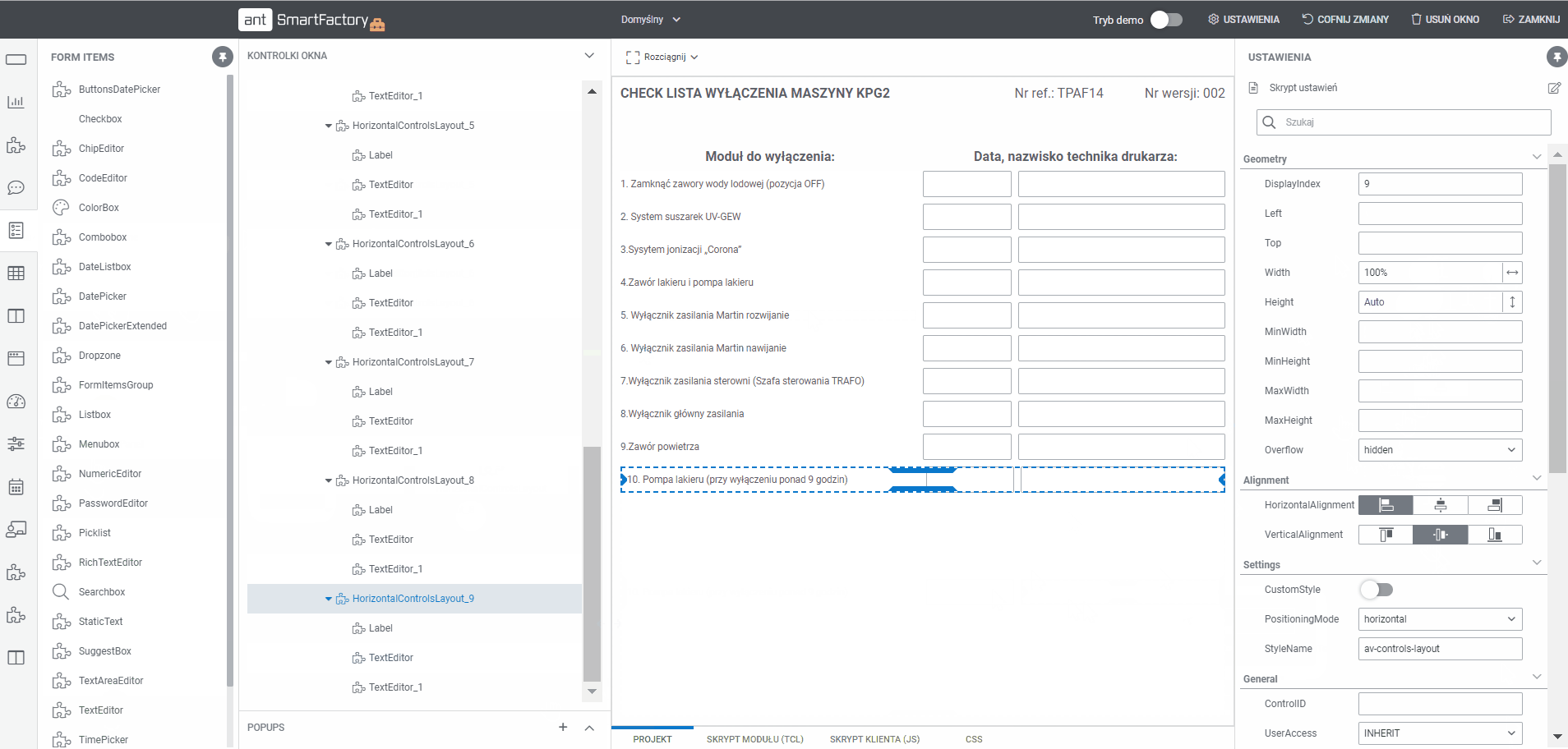
Create documentation MBR templates with Builder
- Display all processes and templates
- Edit the project using a tool that allows you to add sections and drag and drop fields (text, selection, date, label and others)
3.
Use production execution module to:
- Handle the start-up of a production order
- Validate of the process before the start of production
- Guide the operator through the various stages of production with display of analytics
- Automatically register an order fulfilment
- Automatic data capture
- Automatic form filling based on downloaded data
4.
Generate reports in PDF
- Automatically generate a PDF report by the EBR with a summary of the batch release
- Store all generated reports in the system
EBR System - The key features
Digital Documentation
Digital Documentation let users to display documentation online at operator panel. Categorized PDFs, images and movies are being displayed for ongoing production.
EBR - Electronic batch records
Operators fills documentation step by step on the Operator Panel. It is a documentation for the whole manufacturing process in electronic way keeping data integrity. System makes ensure that steps are fully completed with all the required data. The result of EBR creation is the PDF Manufacturing Documentation file.
Traceability
With Traceability tracking and tracing of the finished good, semi-finished good and raw materials is possible. Ongoing production is being matched with S/N, batches of components and containers. The system validates processes and confirms scanned resources. Labelling process is done automatically in any step of production. With Traceability it is possible to find raw material based on scanned finished good and vice versa - after scanning raw material in which finished goods it was used.
MBR – Master batch record
Creating templates of the MBR by setting all activities in the system in the right sequence. Each activity can be configuration with reporting data type like numbers, text, data read from automation, etc. Those values can be validated against quality limits set in the system.
Paperless
Paperless is responsible for digital guidance of operator - starting from displaying activities (list to-do) for production, changeovers with dedicated documentation (image, document, movie) for each step of production. Any paper form can be transferred to the system thanks to Factory Forms.
Quality inspections
Quality Inspections are automatic way for periodic quality tests with immediate confirmation if measurement is within quality limits. Measurements are being read from automation level or are provided manually by operator. Thanks to SPC report and Shewhart control charts the stability of production is being monitored.
Workflow
System guides and monitors the steps of the documentation as a workflow. It helps to find on what phase, the document is now and what actions must be taken to finish the process together with assigned user or its type. Workflow support electronical signature.
System
integration
System integration is responsible for receiving inbound data from ERP and for sending outbound data to any other external system. It works in real-time. Different integration ways are supported like web services, intermediate databases or flat files.
Master
Data
Master Data is a part of the system where production modelling is done. It is a place to specify detailed technology like items, routing, factory structure or production orders.
Master Data
Advanced
Master Data Advanced is extension for standard Master Data. It is to even model processes even deeper including BOMs, activities for paperless step-by-step instructions, labelling, quality limits, setpoints for machines and S/N products.
EBR Software – Integrate with ERP system to enable a complete reporting
The seamless data exchange between enterprise resource planning (ERP) systems and electronic batch records (EBR) system is crucial for optimising production processes and achieving operational excellence.
EBR imports from ERP:
- employees list
- production order data
- sales order information
- order headers
- routings
- BOMs – Bill of materials
- tools list
- item routings with alternative routings
- item BOMs with alternative BOMs
In return outboud interface generates production report with detailed operations:
- operation progress (amount of good and bad pieces reported at each operation of the order)
- scrap details – different scrap reasons with summarized quantities for the order
- bill of materials consumption summary (this can be done but we recommend to consume it via ERP back flushing)
- employees’ work logs – how much time each operator spent on the machine during the production of the particular order
- employees amount during the production of the order
Our EBR integration supports various communication protocols to ensure compatibility with your existing IT infrastructure:
- Web Services (REST, SOAP)
- RFC (SAP)
- Intermediate Database
- Flat Files (XML, CSV)
Implementation of EBR software gives measurable results
EBR software as a way to meet the 10 GMP principles
With MBR templates, procedures, instructions or recipes are well known.
The EBR software enforces the implementation of production processes according to procedures and prevents the use of unauthorised actions keeping data integrity.
EBR uses traceability to check the materials used in production
The system collects information about the ready changeover and gives the green light to start production on the machine in question.
Enforcing adherence to procedures reduces the risk of contamination.
The EBR digitally guides the operator and every important operation must be approved by an authorised person
The EBR checks the next steps and reports them as OK/NOK, this information is available on an ongoing basis to those responsible for control
Standardisation of the workstation is essential to comply with procedures related to sterile production.
All process data are collected and recorded on digital forms, giving access to a full view of the production details of each batch.
With EBR, you know exactly who carried out and controlled a particular production step.
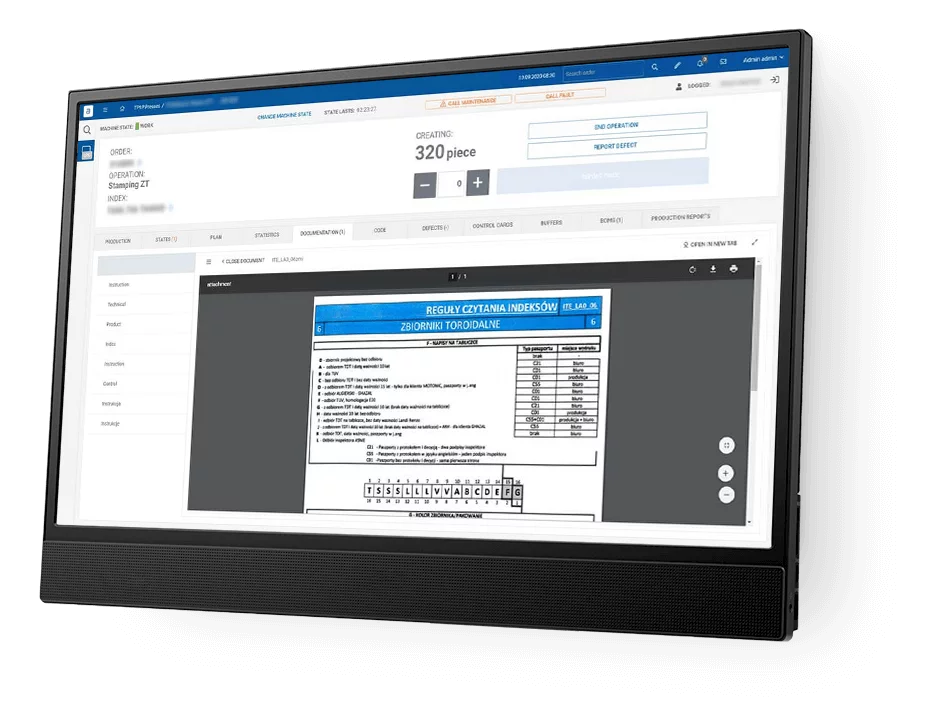
How ANT EBR software differs from other?
Logical and User-Friendly: It’s designed to be straightforward for operators to use. This means that workers can easily navigate through the software without confusion, making their tasks more efficient.
Clear Display: Key steps and values in the Electronic Batch Records software are highlighted and underlined. This feature ensures that critical information stands out, reducing the chances of errors or oversight.
Simplicity: The software includes only the essential entries and signatures required for operations. This approach avoids clutter and makes it easier for users to focus on what’s important.
No Need for Calculations or Interpretations: ANT EBR software does not require operators to perform complex calculations or make subjective interpretations. This reduces the risk of human error and ensures a more consistent and reliable process.
System Presentation
Contact with our Expert

Why to get a demo?
- A 60-minute online meeting with a dedicated specialist presenting a top system from an industry similar to yours
- Live modeling of your production process
- A budget quotation after the meeting
What is more?
LIMS
In the dynamic world of manufacturing, electronic batch records (EBR) have emerged as a game-changer, revolutionizing the way companies produce goods. And when seamlessly integrated with a Laboratory Information Management System (LIMS), the potential for transformative change knows no bounds. For manufacturing professionals seeking to elevate their operations, here’s why exploring EBR in conjunction with LIMS is an opportunity you can’t afford to miss:
- Digitalizing Batch Documentation
- Ensuring Regulatory Compliance and Audit Readiness
- Streamlining Workflows and Process Efficiency
- Enhancing Product Quality and Traceability
- Leveraging Analytics for Continuous Improvement
ALCOA+
ALCOA+ is used to verify that the highest quality and safety standards are met – conditions essential for the correct production of pharmaceuticals. The EBR collects the original data records, thus ensuring that the data taken from the machines during processes is reliable. The ANT system is compatible with the ALCOA+ framework. Every rule is presentable in the system in the event of an audit or inspection. It ensures availability, consistency and endurance along with 6 other factors.
- Attributable: Available information makes it possible to identify who saved the data and when
- Legible: Records must be readable after they are written, permanent, adjusted accordingly (if necessary)
- Contemporaneous: Data must be saved at the time it was generated
- Original: Data source must be accessible, must be preserved intact
- Accurate: Data must accurately reflect the actions/activities performed
- Complete: All required information is included and legible
Related articles

Electronic Batch Record – Automation and Paper Elimination in Pharma
It’s no secret that every step of the pharmaceutical manufacturing process must be documented. However, managing paper documentation can be extremely time-consuming for pharmaceutical manufacturers,

MES in Cosmetics help to reduce waste
Production optimization and related analytics is important for all businesses, regardless of industry. However, there are companies with special characteristics. This group includes, among others,

How to get automatically reports from production
Digitalization means that reporting in its traditional form, as we have known it for years – in paper form, symbolically passed from hand to hand