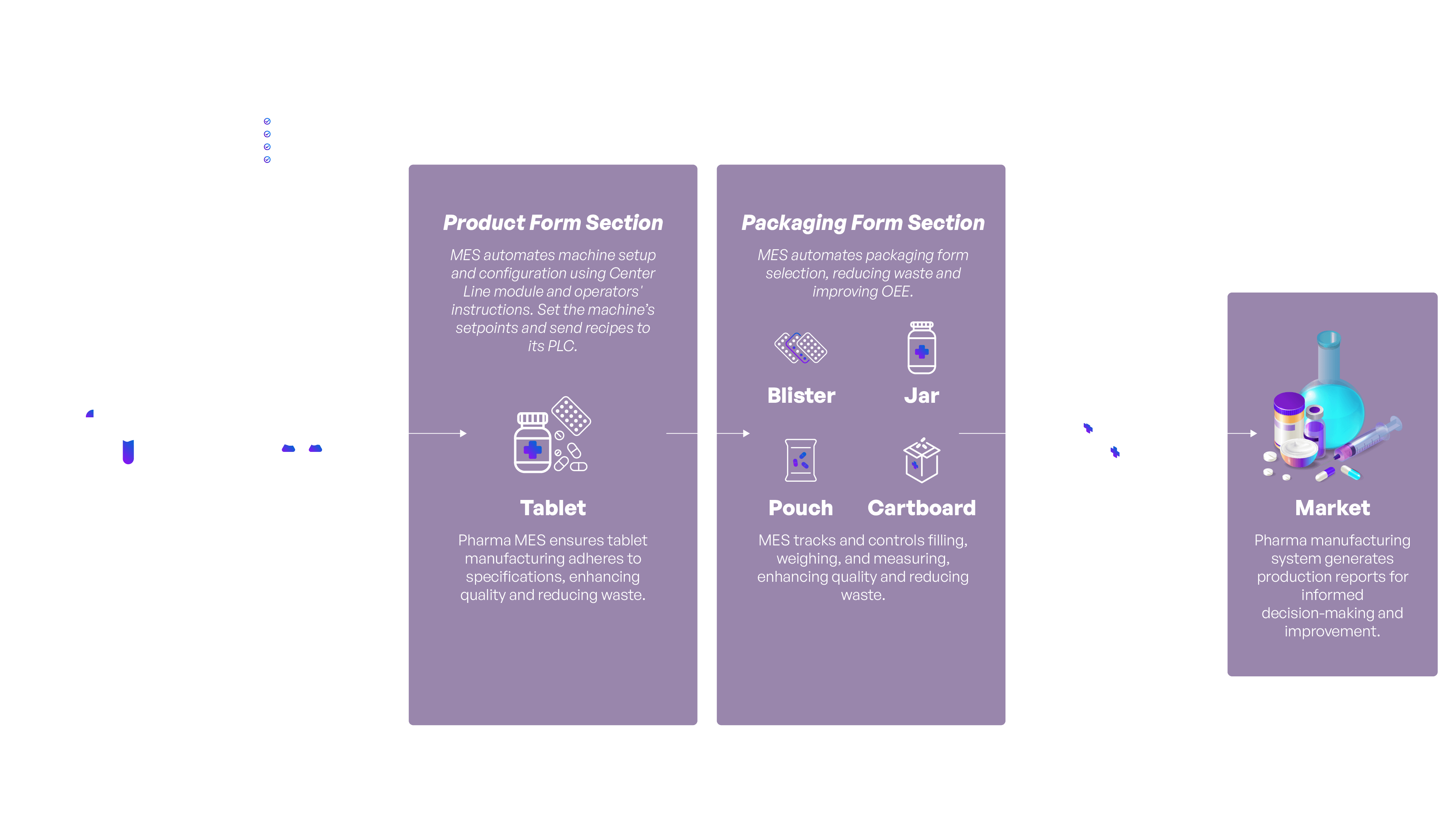
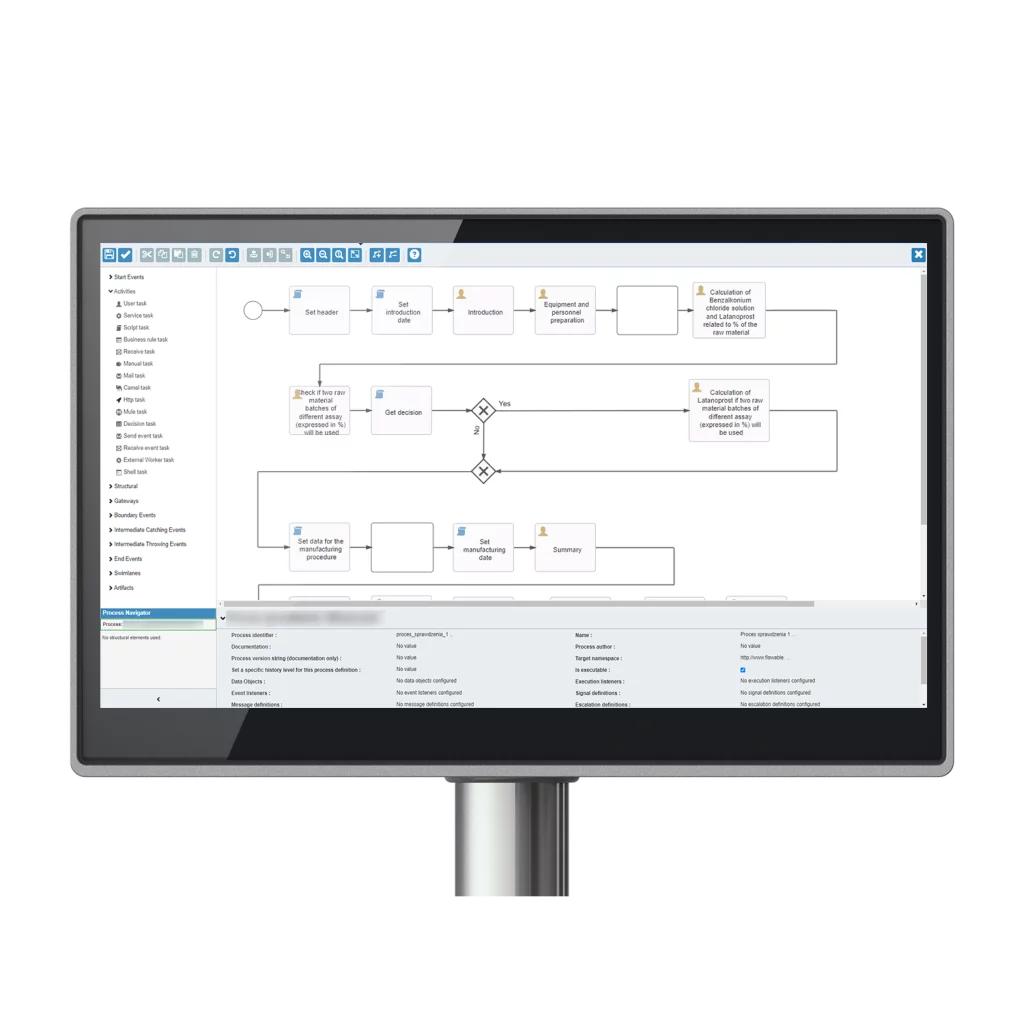
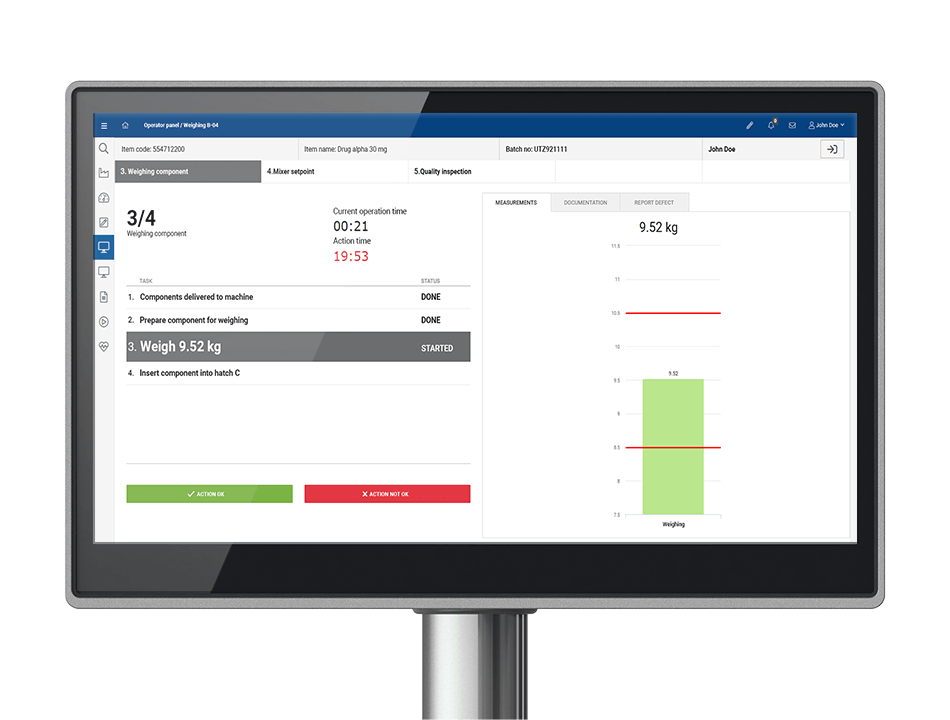
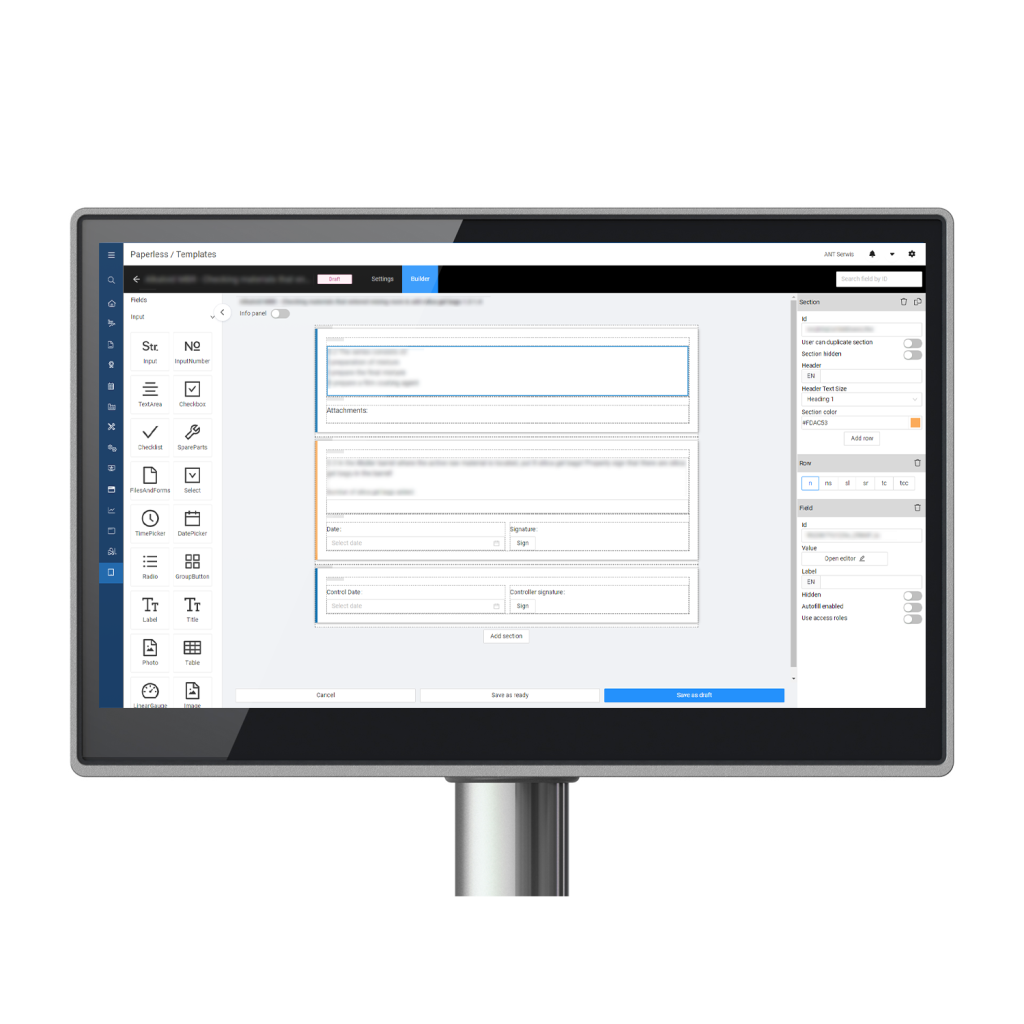
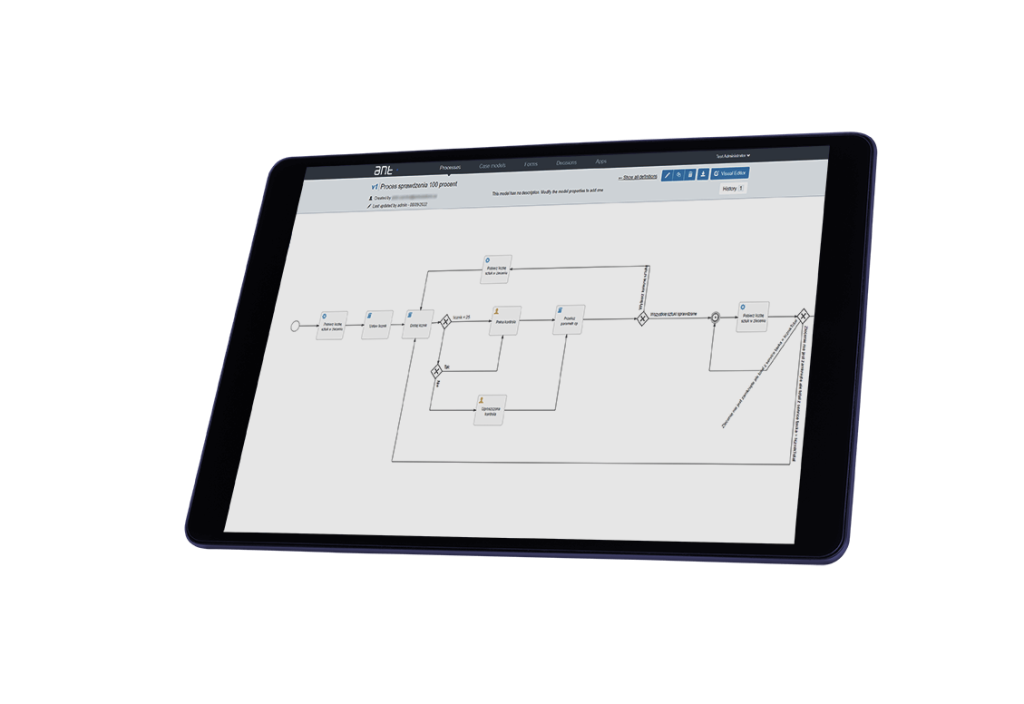
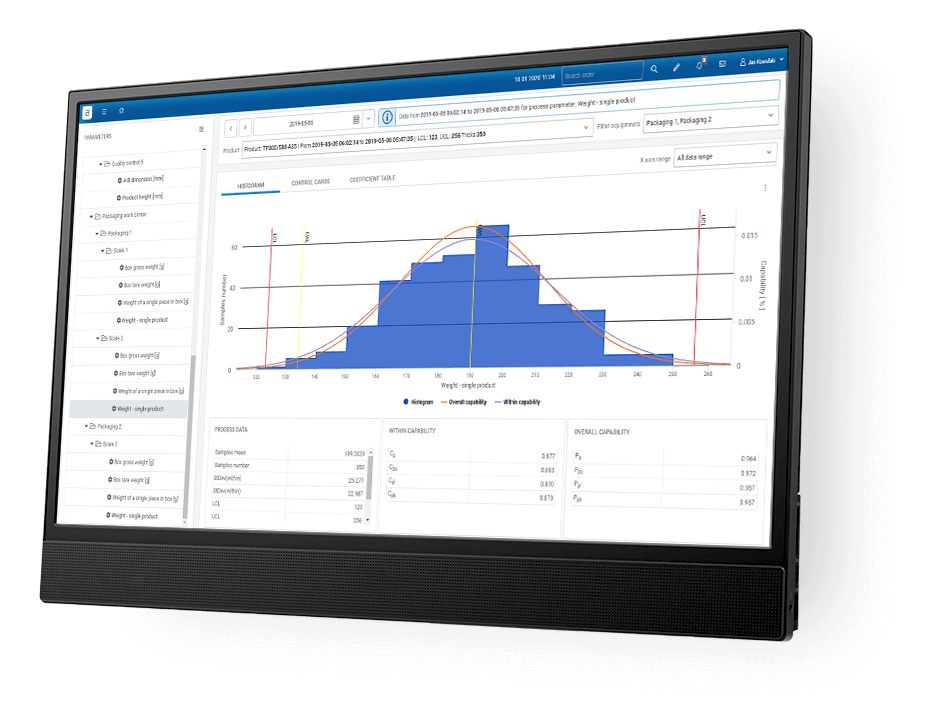
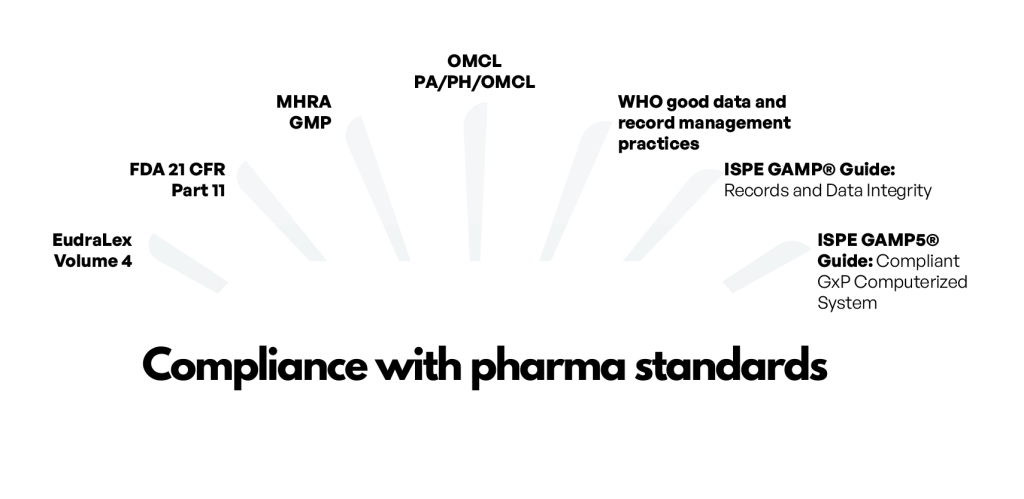
How does MES solution eliminate paper from production and shopfloor?
Digitalization has firmly established itself in everyday production processes, regardless of the industry. Nevertheless, habits remain from the days before widespread digitalization, traditional solutions not