Record del lotto master (MBR) indica un documento che definisce i metodi di fabbricazione, i materiali e altre procedure, le linee guida e i controlli relativi alla fabbricazione e al collaudo dei prodotti, definito anche modello di fabbricazione. L'MBR è parte integrante delle GMP (Good Manufacturing Practice).
Il Master Batch Record è progettato per garantire che vengano aggiunti tutti gli ingredienti corretti e che ogni fase del processo sia completata e documentata.
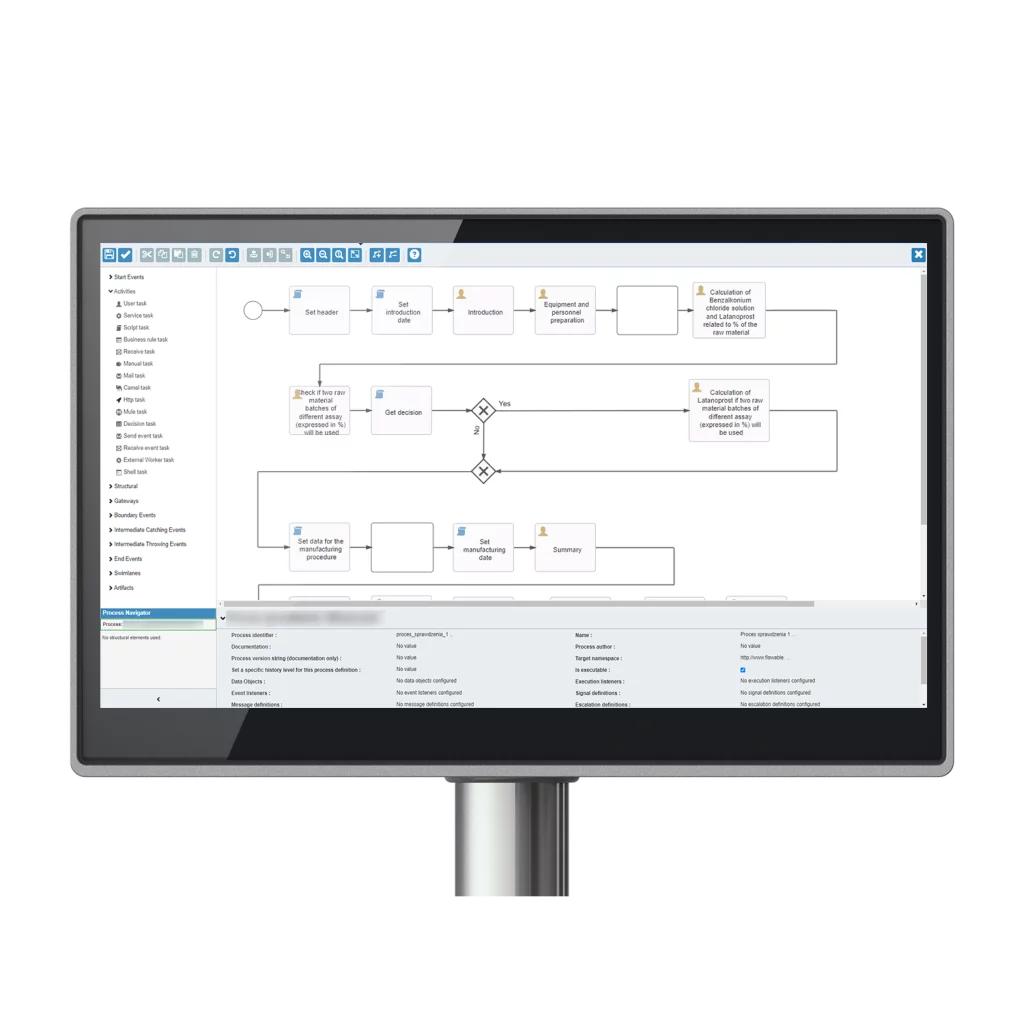
I Master Batch Records MBR (chiamati Master Production Records MPR o Master Manufacturing Formulas MMF) sono istruzioni di produzione dettagliate. Ogni singola ricetta e dimensione del lotto deve avere un proprio MBR..
Il record del lotto principale dovrebbe richiedere le seguenti informazioni, ad esempio:
- Identificazione del nome del prodotto.
- Una distinta dei materiali che indica il peso, la misura o il numero di ogni ingrediente necessario per la produzione del lotto.
- Un elenco di attrezzature.
- Un elenco di componenti.
- Una dichiarazione della resa teorica in ogni fase del processo di produzione.
- La resa prevista del prodotto finito.
- Istruzioni dettagliate per ogni condizione del processo di produzione.
- Procedure di campionamento e analisi.
- Istruzioni per le operazioni manuali.
- Dati di discrepanza
Queste istruzioni documentate sono necessarie per ogni singola ricetta e dimensione del lotto per garantire la standardizzazione e la ripetibilità del processo ogni volta. Poiché l'MBR è il modello originale da cui vengono tratte le copie, non viene compilato durante il processo di produzione. È associato a un numero di produzione o a un numero di parte, ma non a un numero di lotto specifico.
Registro anagrafico computerizzato dei lotti
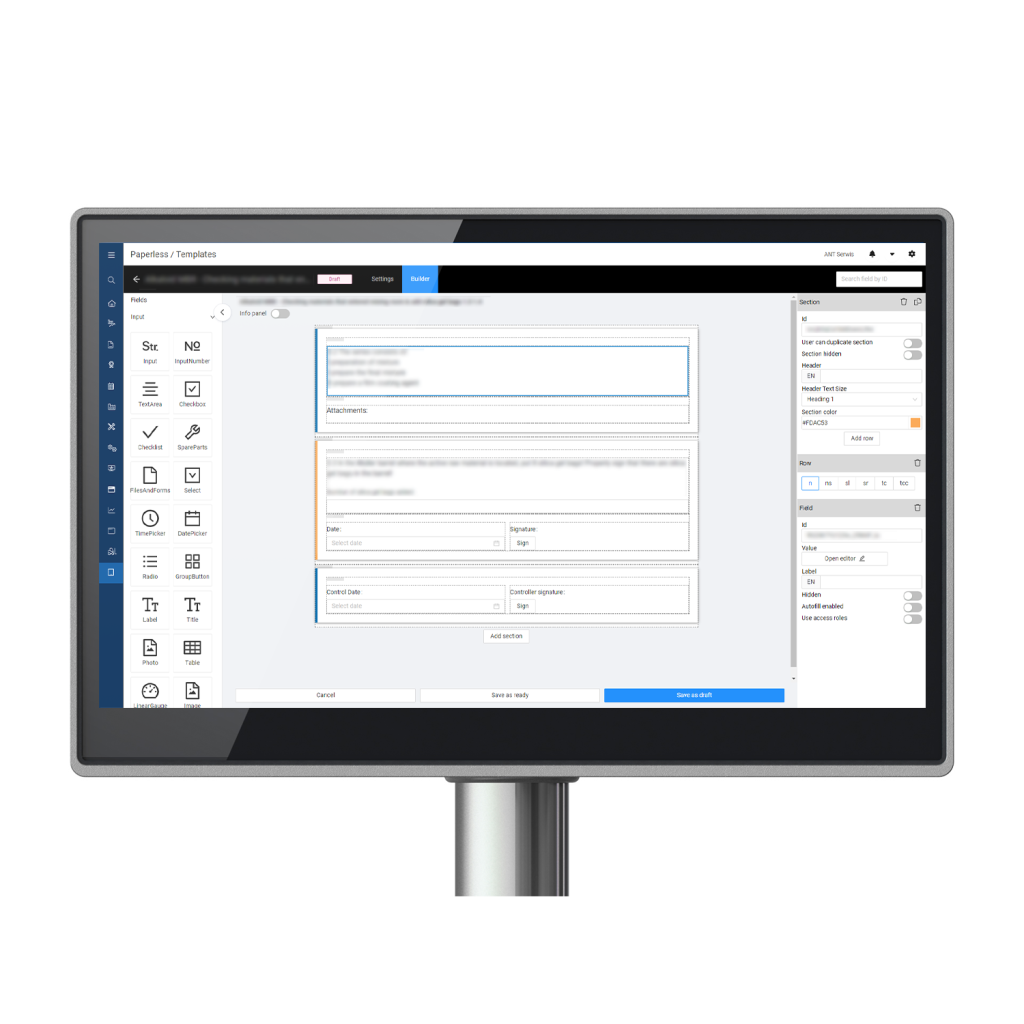
Un Master Batch Record computerizzato è un approccio moderno alla registrazione della produzione, che integra soluzioni software avanzate nel processo produttivo. Questa trasformazione digitale rivoluziona la gestione delle operazioni di produzione, fornendo un sistema completo per creare, gestire e ottimizzare i flussi di lavoro della produzione.
Caratteristiche e vantaggi principali
- Integrazione dei dati in tempo reale: L'MBR digitale si integra perfettamente con diversi sistemi di produzione, consentendo lo scambio di dati in tempo reale. Questa connettività garantisce che informazioni aggiornate siano prontamente disponibili al personale di produzione, migliorando il processo decisionale e la risoluzione dei problemi.
- Riduzione degli errori: L'automazione all'interno di Digital MBR riduce significativamente il rischio di errore umano, automatizzando l'inserimento dei dati e incorporando controlli di convalida. Ciò si traduce in una maggiore coerenza e qualità del prodotto.
- Gestione efficiente delle ricette: L'MBR digitale semplifica la gestione di più ricette per prodotti e lotti diversi. I produttori possono creare, aggiornare e mantenere le ricette senza sforzo, garantendo una produzione sempre accurata.
- Controllo della versione: Le robuste funzionalità di controllo delle versioni dei sistemi DMBR garantiscono la conformità delle istruzioni di produzione alle normative più recenti. I produttori possono adattarsi rapidamente ai cambiamenti dei requisiti senza compromettere la conformità.
- Firme elettroniche e tracce di controllo: L'MBR computerizzato facilita l'apposizione di firme elettroniche e mantiene tracce di audit complete. Questa funzione è fondamentale per tracciare e autenticare le azioni critiche, favorire la conformità normativa e supportare le indagini.